
The number of active cases in the MDL dropped to 769 from 774 the previous month.
This is an Active case
If your baby developed necrotizing enterocolitis (NEC) after consuming certain cow milk-based formulas, you may have a claim.
Connect with an attorney
Parents of premature babies are filing baby formula lawsuits against cow milk-based baby formula brands made by Abbott Laboratories and Mead Johnson. These parents allege that Enfamil and Similac products led their babies to develop necrotizing enterocolitis (NEC), a serious gastrointestinal issue.
Parents of premature infants diagnosed with NEC are filing infant formula lawsuits against Mead Johnson (Mead) and Abbott Laboratories (Abbott). These companies manufacture Similac and Enfamil baby formulas. NEC is a gastrointestinal condition that causes intestinal tissue to die. NEC can lead to serious abdominal and blood infections. In some cases, it may be fatal.
These lawsuits state that Mead and Abbott should have warned consumers about the increased risk of NEC when they feed their cow-milk based formula to premature infants.
Our medical attorneys are reviewing these allegations. If your child has been affected by NEC after consuming formula products, we may be able to help with filing a lawsuit.
Research indicates that cow milk-based baby formulas are more likely to lead to NEC in premature babies. Specific formula products that may be a concern for premature babies include:
Abbott Products | Mead Johnson Products |
|---|---|
|
|
If you use these or other bovine formulas to feed your premature infant, you may wish to speak with your doctor.
Necrotizing enterocolitis (NEC) is a condition that inflames gastrointestinal tissue. This causes tissue in the digestive system to die, which can lead to perforations or tears in the intestinal wall. Weakened intestinal tissue from NEC allows bacteria to leak into the bloodstream and body. This makes infants more susceptible to life-threatening infections.
While any newborn can develop NEC, babies born prematurely develop it more often. NEC affects one in 1,000 preemies and only one in 10,000 full-term infants. It’s also more common in infants with low birth weights, whether premature or not. About 90% of the babies who get NEC are premature or have a low birth weight.
Symptoms of the disease may present differently depending on the infant. Some infants may only experience mild cases and associated symptoms. Other infants have more serious symptoms.
NEC symptoms include:
Seek a doctor’s help immediately if your baby or a baby you care for is showing these symptoms. Babies fed formula or breast milk may develop NEC. However, research at Stanford Medicine Children’s Health says babies not fed breast milk are more likely to deal with NEC.
If your baby has developed NEC after being fed Enfamil or Similac baby formulas, you may have a case. Baby formula lawyers can help you explore a NEC lawsuit.
In addition to the concerns over NEC, Abbott has a history of issues with its baby formula products. In February 2022, Abbott issued a voluntary recall of its Similac, Similac PM 60/40, Alimentum and EleCare baby formula products. The company recalled the products after multiple complaints, which included:
Parents can locate the lot number printed on their baby formula container to determine whether the recall included it. Abbott recalled Similac, Alimentum and EleCare powder formulas with the following lot codes and expiration dates:
Later in February 2022, Abbott also recalled the following Similac PM 60/40 products:
The bacteria reported in these Abbott products can cause serious infections. The U.S. Food and Drug Administration (FDA) followed up both of Abbott’s voluntary recalls with formal recalls of its own.
Symptoms of Cronobacter | Symptoms of Salmonella |
|---|---|
|
|
If you or another caregiver observe these symptoms in your baby, take the baby to a healthcare provider as soon as possible.
In December 2023, the FDA announced that Reckitt/Mead Johnson Nutrition voluntarily recalled certain batches of their Nutramigen Hypoallergenic Infant Formula Powder. These are the affected batches of the baby formula:
If you have any Nutramigen Hypoallergenic Infant Formula Powder with those batch codes, you should not use them. Remove the product to a safe area where it cannot accidentally be used. But, saving the packaging could be an important piece of evidence in a lawsuit. You can also contact Reckitt/Mead Johnson Nutrition for a refund.
The FDA has not received reports of illnesses related to this Nutramigen recall. Public health agencies in other countries detected a pathogen in the formula, Cronobacter sakazakii, which prompted the recall.
Cronobacter sakazakii occurs naturally. It can enter factories and homes via hands, shoes and other contaminated surfaces.
Parents are filing lawsuits against baby formula makers Mead Johnson and Abbott Laboratories for failing to warn them about the risk of NEC when premature babies are fed their cow-milk based formulas.
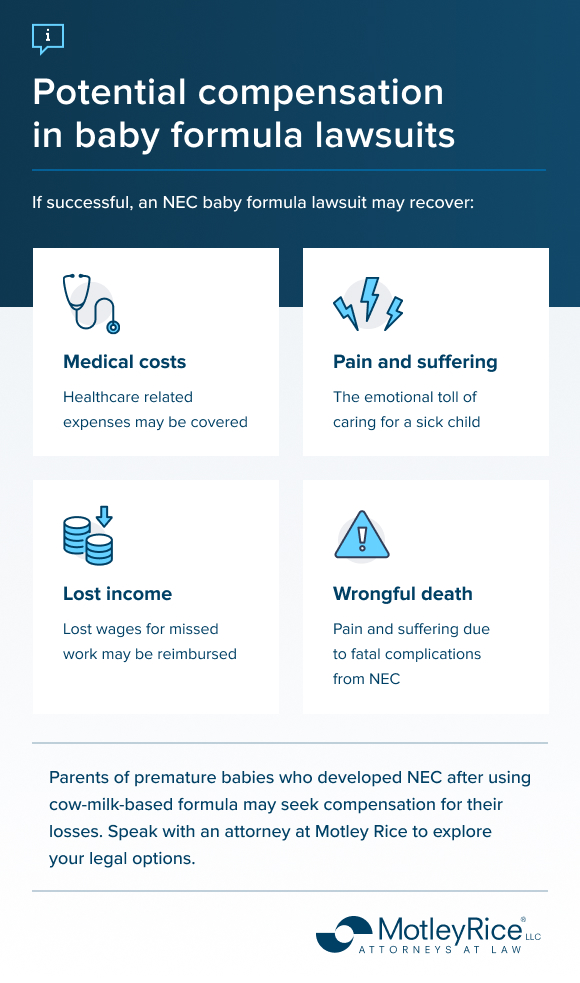
These lawsuits include multiple products across the Enfamil (by Mead Johnson) and Similac (by Abbott) brands. Parents/guardians are seeking compensation for their emotional distress, the harms caused to their infants and financial losses associated with NEC.
If you have a premature infant who was diagnosed with NEC after being fed Similac or Enfamil products, you may be eligible to file a baby formula lawsuit.
No, the NEC baby formula cases aren’t a class action lawsuit. They are a similar type of mass tort lawsuit called multidistrict litigation (MDL). There is a separate baby formula class action lawsuit for issues related to Abbott’s recall. However, this is not connected to the NEC baby formula lawsuits discussed here.
Most of the NEC lawsuits are collected in an MDL docket in the U.S. District Court for the Northern District of Illinois. This doesn’t mean parents have to live in Illinois to file a lawsuit. MDLs organize multiple lawsuits against the same defendants into one court. Consolidating lawsuits can help establish common facts and expedite the legal process.
The NEC baby formula lawsuits are a part of In re: Abbott Laboratories, et al., Preterm Infant Nutrition Products Liability Litigation, MDL No. 3026. As of January 5, 2026, there were 769 pending actions in the docket. Many other cases are filed in similar consolidations in various state courts, including Missouri, Illinois and California.
Motley Rice baby formula attorneys may be able to help you file a Similac or Enfamil lawsuit. We have experience helping plaintiffs fight for justice against the manufacturers of defective products.
A toxic baby food lawsuit is currently organized as an MDL. It is unrelated to the NEC baby formula lawsuit, but the two can be confused because of their similar names. In the baby food lawsuit, plaintiffs allege that major baby food manufacturers knowingly manufactured and sold baby foods containing toxic heavy metals. Defendants include:
The baby food and baby formula mass torts are not related.
Next is a summary of key milestones and updates in the NEC infant formula lawsuits.
1.05.26
The number of active cases in the MDL dropped to 769 from 774 the previous month.
12.03.25
New plaintiffs continued to join the baby formula MDL throughout MDL. The total number of pending cases is now 774.
11.03.25
The Judicial Panel for Multidistrict Litigation (JPML) reported that there are now 755 plaintiffs in the baby formula MDL.
10.01.25
There are now 761 lawsuits in the baby formula MDL.
09.02.25
The Baby Formula NEC MDL now includes 760 pending actions.
08.01.25
There were 759 pending actions in the Baby Formula NEC MDL leading into August.
07.02.25
There were 744 pending actions in the Baby Formula NEC MDL.
06.03.25
The Baby Formula NEC MDL now includes 740 pending actions.
05.02.25
Early in May, the Illinois federal judge overseeing the MDL issued a summary judgment in favor of defendant Abbott Laboratories. The case was supposed to head to trial as the first bellwether case just a few days later.
05.02.25
There were 710 pending actions in the Baby Formula NEC MDL.
04.02.25
Cases continued to grow as 20 new claims were added to the MDL, bringing the total to 683 in April 2025.
03.13.25
A Missouri state court judge overturned an October 2024 defense verdict that cleared Abbott and Mead of liability. The judge cited misconduct by the defense attorneys as the basis for vacating the verdict and ordering a new trial. The misconduct cited included intentional violations of court orders and misleading the jury.
03.03.25
There were 663 pending actions in the Baby Formula NEC MDL. This marked an increase of 15 cases from February 2025.
02.04.25
The Baby Formula NEC MDL has increased to 648 pending cases.
01.03.25
The Baby Formula NEC MDL has increased to 632 filed claims.
12.01.24
With the addition of two more cases, the NEC baby formula MDL has grown to 626 active cases.
11.01.24
There are now 624 NEC baby formula lawsuits pending in the MDL. Plaintiffs may continue to file suit to join this consolidated litigation.
10.31.24
The jury sided with the defendants, Abbott and Mead Johnson, in the case brought by Elizabeth Whitfield.
10.04.24
The first four bellwether trials were scheduled for the following dates: May 5, 2025, August 11, 2025, November 3, 2025, and February 2, 2026. These were proposed trial dates still waiting on court approval.
10.01.24
The NEC baby formula multidistrict litigation (MDL) had 598 cases as of the beginning of October 2024. This included an additional 200 cases filed in federal court since January 2024.
09.30.24
A jury trial against multiple defendants, including Abbott and Mead Johnson, began in Missouri on September 30. Elizabeth Whitfield, the plaintiff in the trial, alleged that her son, born prematurely at less than 28 weeks old, developed NEC after consuming cow milk-based formula. The lawsuit alleged that the companies and the hospital failed to warn the plaintiff about the risks. The condition required surgery for treatment and caused potentially life-long suffering for the plaintiff’s child.
08.01.24
The NEC mass tort had 538 baby formula cases pending in MDL No. 3026 as of the start of August 2024.
07.31.24
The first trial against Abbott Laboratories began in Missouri. This had the potential to serve as a bellwether trial for the MDL.
07.26.24
A jury awarded $495 million, including $400 million in punitive damages, to an Illinois woman. She alleged that Abbott’s premature infant formula was responsible for her infant daughter’s NEC diagnosis.
07.01.24
534 baby formula cases were pending in MDL No. 3026, which is the NEC mass tort.
03.01.24
An Illinois jury awarded $60 million to the mother of a baby who died from intestinal disease. The jury believed Mead Johnson failed to properly warn parents of the risk of NEC from its milk-based products. The plaintiff is not represented by Motley Rice.
01.01.24
The MDL reached 342 actions pending at the beginning of the year.
12.01.23
Reckitt/Mead Johnson Nutrition voluntarily recalled batches of Nutramigen Hypoallergenic Infant Formula Powder that may be tainted by the Cronobacter sakazakii pathogen. This recall is not a part of the NEC baby formula lawsuits but is important information for worried parents.
04.01.23
The Multidistrict Litigation Panel (JPML) transferred NEC lawsuits to an MDL docket. The panel appointed Judge Rebecca Pallmeyer to oversee the litigation in the U.S. District Court for the Northern District of Illinois.
02.01.22
Abbott voluntarily recalled Similac, Similac PM 60/40, Alimentum and EleCare baby formula products. This recall was not a part of the NEC baby formula lawsuits but was important information for worried parents.
There is no proposed settlement for the NEC baby formula lawsuits. As of October 2025, the MDL docket is still active, and cases are proceeding. It’s difficult to put an exact timeline on mass tort litigation.
NEC baby formula lawyers continue to examine the applicable laws and scientific evidence. Many law firms are still accepting new cases in the MDL against baby formula manufacturers.
State governments set the statute of limitations for product liability lawsuits such as the NEC infant formula lawsuits. Some states set a statute of limitations as short as one year, while others might be six years.
If you believe tainted baby formula caused your child to develop NEC, contact a baby formula attorney. They can provide a case evaluation to see if you’re within your state’s statutes of limitations.
You might be eligible for financial compensation from a NEC infant formula lawsuit if your situation matches certain criteria. Typical eligibility requirements include:
There is no set award or settlement amount for NEC lawsuits. Lawsuit awards depend on the judge and jury, and they take the facts of the case into account. Settlement offers for plaintiffs will also vary depending on the nature of the injuries. In the first case to go to trial in March 2024, a jury awarded a $60 million verdict to a mother whose baby died from NEC. In July 2024, a jury awarded $495 million to an Illinois mother whose infant survived NEC but needed long-term care. These plaintiffs were not represented by Motley Rice.
Motley Rice attorneys have worked for decades to seek justice for children and families living with the lifelong effects of toxic and dangerous exposures.
The firm’s additional experience includes:
Learn more about our experience with toxic exposure lawsuits.
Do not stop taking a prescribed medication without first consulting with your doctor. Discontinuing a prescribed medication without your doctor's advice can result in injury or death.
Important NEC lawsuit updates
Key takeaways
Why are people filing baby formula lawsuits?
What is NEC?
Abbott baby formula recall
Mead Johnson formula recall
Necrotizing enterocolitis lawsuit status
Toxic baby formula lawsuit updates
Frequently asked questions about NEC baby formula lawsuits
Our experience fighting for families and children
Call us or fill out our online form with the details of your potential case.
Our team reviews your information to assess your potential case.
Talk with us about next steps.